Mini Organs Pave the Way for Understanding Gastroesophageal Cancer
Posted
30 Nov 22
USC researcher Dechen Lin has created miniature organs — with the hope of fighting a rare type of cancer.
CANCERS THAT IMPACT THE JUNCTION of the esophagus and the stomach are rare — but debilitating. They get less attention than breast, prostate or lung cancers — and 85 percent of patients diagnosed with gastroesophageal junction tumors die within five years. According to the National Cancer Institute, gastroesophageal cancers kill more than a million people every year around the world, and the type of cancer has become more common in recent decades.
“The clinical need is pretty unmet,” says Assistant Professor Dechen Lin. “And the situation has been dire for decades.”
Cancer in this area is challenging to study — mice, which are the typical study model for cancer — don’t have the same anatomy between their esophagus and stomachs. Another option is growing cells from a patient in the lab — but those cells form a flat surface, lacking the fuzzy structures that absorb nutrients and other three-dimensional contours of the region.
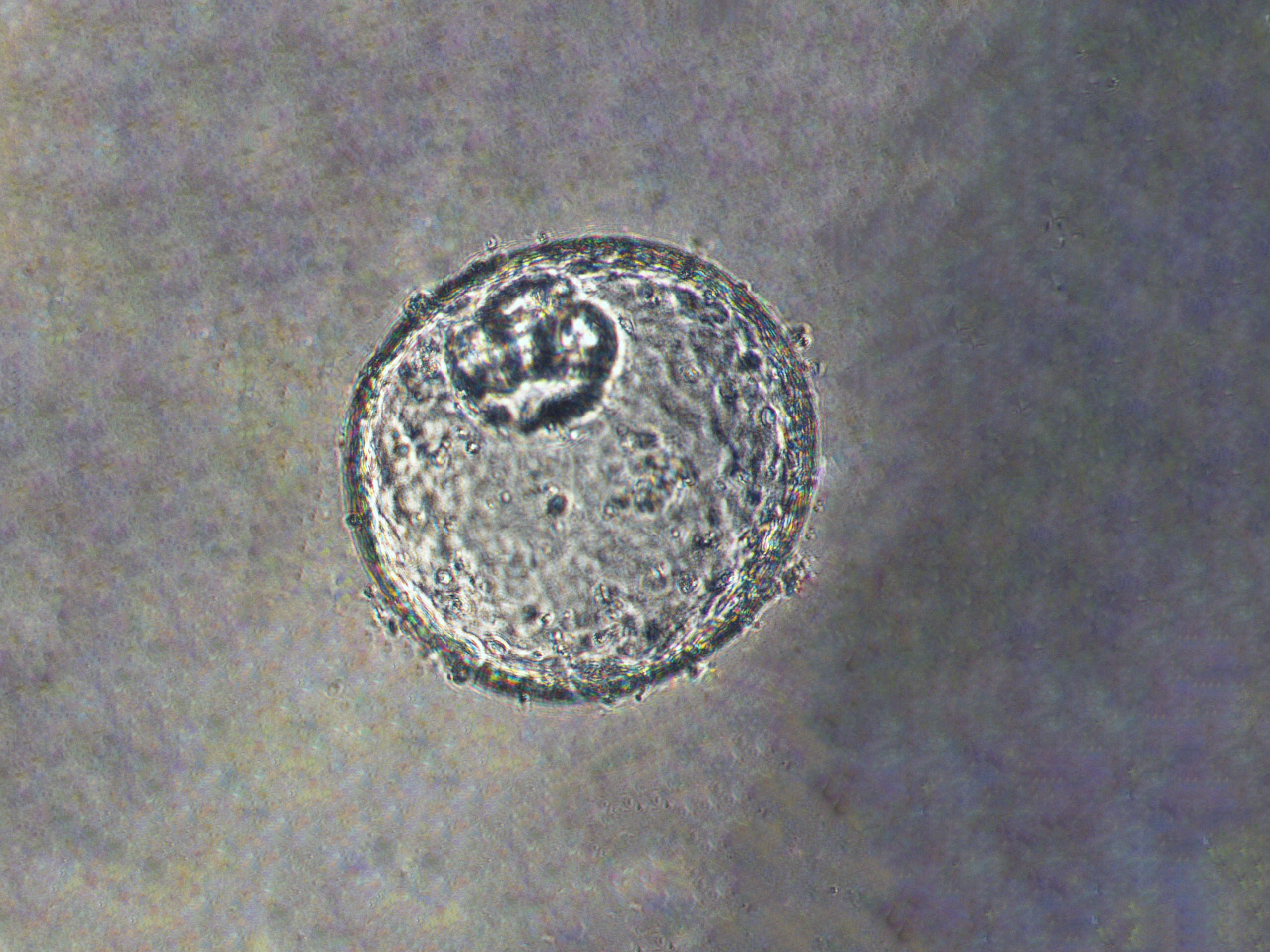
A New Model
That’s why Lin, in collaboration with a lab at Johns Hopkins University, decided to create an organoid — a new model for studying the disease that grows in three dimensions and functions as the organs would in a real patient.
It took about three years to coax the pea-sized clump of cells to develop under special growth conditions embedded in a gel in a dish, Lin says. They had to monitor the organoid’s pH, levels, nutrients and even the stiffness of the gel — figuring it out as they went along.
But when they finally succeeded in creating the organoid in a reproducible way, the model gives researchers a new way of understanding gastroesophageal junction cancer. “Once it’s established, it’s tremendously helpful, because it has everything to do with human anatomy,” Lin says.
Described in a new study, published today in the journal Science Translational Medicine, Lin and his colleagues used genome editing technology to disrupt two key genes in the organoids. When the researchers knocked out those genes, the gastroesophageal cells became larger in size, growing more rapidly, and appearing closer to malignancy. These altered organoids were able to grow tumors in immunodeficient mice.
One factor that puts people at risk of gastroesophageal cancer is obesity — it makes the cancer five to ten times more likely to occur. Obese people have a more dysregulated repertoire of lipids. In the organoids, the researchers noticed alterations in a particular type of lipids — especially one called platelet activating factor, which stores energy. Because of this, they tested a compound for treating platelet diseases, and it potently slowed the growth of the tumors in mice.
The work has implications for treating people with cancer, but also for understanding its basic biology, Lin says. “We’re doing basic translational research, which is very tedious and takes a lot of time,” he says. “Although we don’t produce drugs in the labs every day, what we are learning and what we’re finding are the bedrocks for the future therapies and clinical management for cancer patients.”